Early-onset neural disorders (EOND) encompass a range of conditions that affect the nervous system. They often show up in childhood or adolescence. The causes can be complex. However, in all cases, genetics plays a crucial role.
Multiple factors influence the development of these diseases, including:
- Single-gene mutations
- Multiple-gene interactions
- Chromosomal abnormalities
Understanding the impact of genes on EOND is essential. It’s vital for evaluation and medical approaches. It improves the lives of those affected by these diseases. In our article, we will take a closer look at the genetics side, role of mutations, biomarkers and genetic research.
What Are Early-Onset Neurological Disorders and Why Genetics Matter?
These encompass a wide spectrum of conditions. These disorders affect the human nervous system and typically manifest symptoms during childhood or adolescence. These disorders can impact many aspects of neural function, including motor skills, mental abilities, behavior, and sensory perception.
Genetics plays a central role in the development of these diseases. Our genetic makeup, inherited from our parents, provides the blueprint for our bodies. It includes the intricate workings of the nervous system. Variations or errors in this genetic code can disrupt the normal development and function of the nervous system. It can lead to neural disorders (ND).
Genetic mutations are a primary cause of EOND. These issues can occur spontaneously or be inherited from a parent. Some of them may have a direct and immediate impact. They can cause neural symptoms to appear early in life. Other ones may increase the risk of developing an ND later in life. However, the onset is still considered early if it occurs during childhood or adolescence.
Hereditary Diseases and Their Influence on Neurological Health
These diseases are also known as genetic ones. They’re passed down from parents to their children through genes. These issues can significantly contribute to EOND. If a parent carries a genetic mutation, their child may inherit it and develop the condition.
Several specific mutations are responsible for many EOND. For instance, mutations in the gene responsible for producing the protein dystrophin can lead to Duchenne muscular dystrophy. It’s a progressive muscle-wasting disorder that often manifests in early childhood. And mutations in the gene for producing the protein huntingtin can cause Huntington’s disease. It’s a disease that typically appears in adulthood but can also have an early-onset form.
The Role of Genetic Mutations in Neurodevelopment and Disease Progression
Mutations can disrupt neurodevelopment in many ways. It leads to the onset of neural symptoms. Some may interfere with the formation of nerve cells, their connections, or the production of essential transmitters. These can occur during fetal development, childhood, or adolescence. It depends on the specific anomaly and its impact.
The connection between genetic anomalies and the onset of neural symptoms can be complex. Some anomalies may cause immediate and severe impairments. Meanwhile, others may have a more subtle effect. They can lead to effects that gradually worsen over time. The timing and severity can also vary. It depends on environmental influences and the presence of other gene variations.
Understanding the role of genetic mutations in neurodevelopment and disease progression is crucial. It’s vital for developing effective treatment for EOND. By finding the specific genes and anomalies involved in these conditions, doctors can gain insights into the underlying mechanisms of the issue and develop targeted treatment.
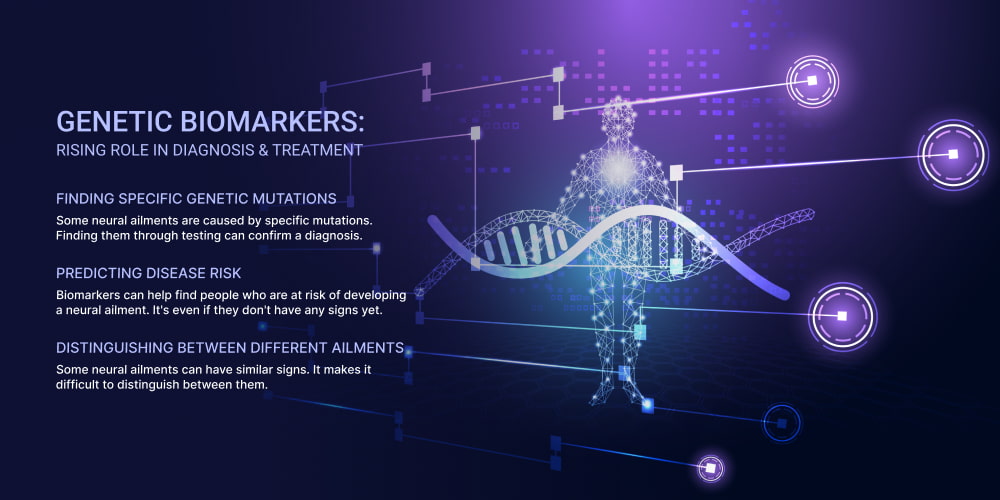
The Growing Importance of Genetic Biomarkers in Diagnosis and Treatment
DNA markers are specific DNA sequences or variations. They can be used to find and evaluate diseases, predict risk, and guide treatment decisions. In EOND, DNA markers play a crucial role in diagnosis and treatment.
These markers can help diagnose EOND in several ways:
- Finding Specific Genetic Mutations. Some diseases are caused by specific mutations. Finding them through testing can confirm a diagnosis. It’s even before symptoms appear.
- Predicting Disease Risk. Biomarkers can help find people who are at risk of developing a ND. It’s even if they don’t have any effects yet. This information can help guide preventive measures and early interventions.
- Distinguishing Between Different Types. Some diseases can have similar features. It makes it difficult to distinguish between them. DNA markers can help differentiate between them.
Finding genetic markers can lead to personalized treatment plans. They’re tailored to an individual’s specific condition and genetic makeup. This is because different genetic variations can affect how a person responds to certain treatments.
For example, people with epilepsy. They may have a variation that makes them more likely to respond to a particular anti-seizure medications. By finding this variation, doctors can choose the most effective medication. It increases the chances of successful treatment and reduces the risk of side effects.
Advancements in testing and screening have made it easier and more affordable to find biomarkers. These advancements include:
- Next-Generation Sequencing (NGS). This is a powerful technology that can sequence large amounts of DNA quickly and accurately. This has made it possible to find a wide range of genetic variations associated with neurological conditions.
- Genome-Wide Association Studies (GWAS). They’re large-scale studies that compare the DNA of people with and without a particular disease with the goal of identifying genetic variations associated with the disease. GWAS have been instrumental in finding new DNA markers for diseases.
- Liquid Biopsies. They’re a non-invasive way to collect DNA from blood or other bodily fluids. This can be used to find DNA markers in people who are unable to undergo more invasive procedures.
Advances in Genetic Research: Unlocking New Treatments for Early-Onset Neurological Disorders
EOND presents significant challenges due to their early impact on development. However, groundbreaking advances in DNA research are offering hope for new treatments. Researchers are finding the specific mutations responsible for these diseases. It paves the way for targeted treatments.
One crucial area involves understanding how these anomalies disrupt normal neural function. For instance, studies are delving into the role of:
- Gene expression
- Protein misfolding
- Cellular signaling pathways
These studies focus on infantile epilepsy, childhood-onset Parkinson’s, and many forms of muscular dystrophy. Scientists pinpoint the precise mechanisms affected by faulty genes. This allows them to develop interventions aimed at correcting or compensating for these disruptions.
Ongoing clinical trials are evaluating the safety and efficacy of several innovative treatments. Gene therapy is a promising avenue. It involves delivering corrected or functional copies of the mutated gene into the patient’s cells. This approach holds immense potential for conditions where the underlying problem is a missing or non-functional protein.
Another strategy is RNA interference. It aims to silence or reduce the expression of harmful genes, proving useful in diseases caused by overactive or toxic proteins. Furthermore, researchers are investigating the use of CRISPR-Cas9 gene editing. It’s needed to directly correct mutations within the DNA. It’s still largely in its early stages. However, it holds immense promise for permanently fixing the root causes.
The future of treatment for early-onset NDs is bright. Our understanding of the human genome deepens, and technology advances. However, we can expect to see more targeted and effective therapeutic options in the future.
How Understanding Genetics Can Help Families Plan for the Future
Understanding genetics plays a crucial role in helping families plan for the future. It’s especially when there’s a risk of EOND. Counseling provides invaluable support to families navigating these issues.
Counseling is a process that involves a trained professional. For example, a genetic counselor or medical geneticist. These professionals help families understand and adapt to their new circumstances. Counseling offers several benefits:
- Risk Assessment. Counselors can assess the risk of a child inheriting a genetic condition based on family history and testing. They can explain the different inheritance patterns and the likelihood of recurrence.
- Education. Counselors provide comprehensive information about specific diseases. It includes their causes, effects, prognosis, and available treatments. They can help families understand complex information and make informed decisions.
- Testing Options. Counselors can discuss available testing options. It includes carrier, prenatal testing, and preimplantation genetic diagnosis (PGD). They can explain the benefits and limitations of each test. And they can help families decide which tests are appropriate for their situation.
- Emotional Support. Receiving a diagnosis of a hereditary disease or learning about the risk of inheritance can be emotionally challenging. Counselors provide emotional support and guidance to families. They help them cope with the information and make informed decisions.
- Family Planning. Counseling can help families explore different family planning options. For example, adoption, donor eggs or sperm, or in vitro fertilization (IVF) with PGD if they’re at high risk of passing on a genetic condition.
Understanding the genetic basis of ND can open doors to intervention strategies. In some cases, knowing the specific mutation allows for early monitoring and interventions to reduce or delay the onset of symptoms. For example, in some metabolic diseases that can cause neural damage. In these cases, early dietary interventions can significantly improve outcomes. Research is also exploring preventative therapies for some genetic conditions.












Please, leave your review
Write a comment: