Long COVID, also known as post-acute sequelae of SARS-CoV-2 infection (PASC), is characterized by persistent symptoms extending beyond the acute phase of COVID-19. This condition affects up to 30% of survivors, including those who experienced only mild initial illness. The nervous system is particularly vulnerable, with neurological complications reported in 10-20% of cases.
SARS-CoV-2 can invade neural tissues via ACE2 receptors, triggering neuroinflammation through cytokine storms involving IL-6 and TNF-α. This process leads to microvascular damage, hypoxia, and potentially autoimmune conditions that attack neurons and myelin. Central nervous system effects include reductions in gray matter in the hippocampus and frontal lobes visible on MRI, while peripheral involvement manifests as small-fiber neuropathy. Autonomic dysregulation disrupts the control of heart rate and blood pressure.
A hallmark symptom is long COVID brain fog, which encompasses cognitive slowing, memory difficulties, and poor concentration. This condition severely impairs daily function and work capacity, significantly reducing quality of life and highlighting the urgency of early intervention to prevent irreversible neural damage.
Neurological Long COVID Symptoms: Focus on Brain Fog, Dizziness, and Neuropathy
Neurologically, long COVID affects the brain and nerves long after viral clearance, impacting 20-40% of survivors. Brain fog is the most commonly reported symptom: patients struggle to focus, recall words, or plan simple tasks. Dizziness disrupts daily activities, with standing triggering lightheaded spells or vertigo. Neuropathy causes burning sensations in the hands and feet, accompanied by pins-and-needles pain.
Common neurological symptoms include:
- Brain fog: Poor concentration, memory gaps, and mental fatigue following even minor efforts
- Dizziness: Orthostatic hypotension, vestibular imbalance, and chronic unsteadiness
- Neuropathy: Small-fiber burning sensations, large-fiber numbness, and tingling in the extremities
- Headaches: Migraine-like throbbing and tension-type headaches
- Sleep disturbances: Unrefreshing sleep and vivid nightmares
- Sensory changes: Loss of smell and taste, heightened pain sensitivity
These symptoms often overlap and worsen with exertion. fMRI scans reveal reduced activity in the frontal and temporal lobes. Persistent inflammation and elevated cytokines irritate neurons, while reduced blood flow from microthrombi compounds the problem. Autoantibodies may target nerve sheaths, and vestibular testing often shows inner-ear damage. Skin biopsies confirm reduced nerve fiber density. Women under 50 report more severe symptoms, which typically peak around six months post-infection but can persist for years. Most patients experience gradual improvement, although approximately 15% continue to experience persistent impairment.
POTS After COVID and Mechanisms of Autonomic Dysfunction
Postural Orthostatic Tachycardia Syndrome (POTS) has emerged as a significant complication in long COVID patients, affecting 2-14% of cases. POTS is characterized by a heart rate increase of 30 beats per minute or more upon standing, without a corresponding drop in blood pressure. Symptoms of POTS after COVID include dizziness, palpitations, and profound exhaustion. The syndrome typically develops 3-12 months post-infection and disproportionately affects young women.
Primary mechanisms of POTS after COVID include:
- Viral invasion: SARS-CoV-2 infects autonomic ganglia and disrupts vagus nerve signaling
- Autoimmunity: Antibodies target adrenergic or muscarinic receptors, causing dysregulation
- Hypovolemia: Increased vascular permeability reduces blood volume, straining cardiovascular function
- Small-fiber damage: Damage to unmyelinated nerves impairs vascular tone, leading to blood pooling in the legs
- Cytokine elevation: Elevated IL-6 and TNF-α increase norepinephrine levels
- Baroreflex dysfunction: Impaired feedback mechanisms result in inappropriate tachycardia
- Genetic factors: Norepinephrine transporter gene variants impair catecholamine clearance
Tilt-table testing confirms the diagnosis. Blood tests can detect relevant autoantibodies, and neuroimaging may show brainstem abnormalities. Treatment includes beta-blockers to control heart rate, increased fluid and salt intake to expand blood volume, and the use of compression stockings to prevent venous pooling. Gradual exercise reconditioning helps rebuild orthostatic tolerance. IVIG shows promise for autoantibody-mediated disease. Recovery is often prolonged, with approximately 30% of patients experiencing symptoms for two years or longer. Early intervention can prevent cardiac complications.
Post-Viral Dysautonomia and Long-Term Patient Effects
Post-viral dysautonomia can develop following various infections, including COVID-19, Epstein-Barr virus, and influenza. The autonomic nervous system fails to regulate heart rate, digestion, body temperature, and pupillary responses properly. In long COVID, dysautonomia affects 30-50% of patients with neurological complications. The long-term effects are profound: severe fatigue confines many patients to limited activity, approximately half of severely affected patients remain unable to work two years after onset, and depression rates double.
Here are the post-viral dysautonomia core impacts:
- Orthostatic intolerance: Syncope risk and persistent lightheadedness
- Gastroparesis: Nausea, bloating, and early satiety
- Bladder dysfunction: Urgency, urinary retention, and recurrent infections
- Thermoregulatory failure: Excessive sweating, chills, and heat intolerance
- Pupillary abnormalities: Photophobia and blurred vision
- Cardiovascular strain: Persistent tachycardia and palpitations
- Sleep disruption: Non-restorative sleep and frequent nightmares
While some patients recover within 1-2 years, 20-30% experience persistent symptoms. Medications such as ivabradine can reduce heart rate, while pyridostigmine enhances autonomic nerve signaling. Energy conservation strategies are essential. Multidisciplinary clinics monitor cardiovascular, gastrointestinal, and neurological function. Early treatment can prevent some permanent complications. COVID-19 vaccination helps prevent new episodes in susceptible individuals.
Diagnosis Methods and When to Consult a Neurologist
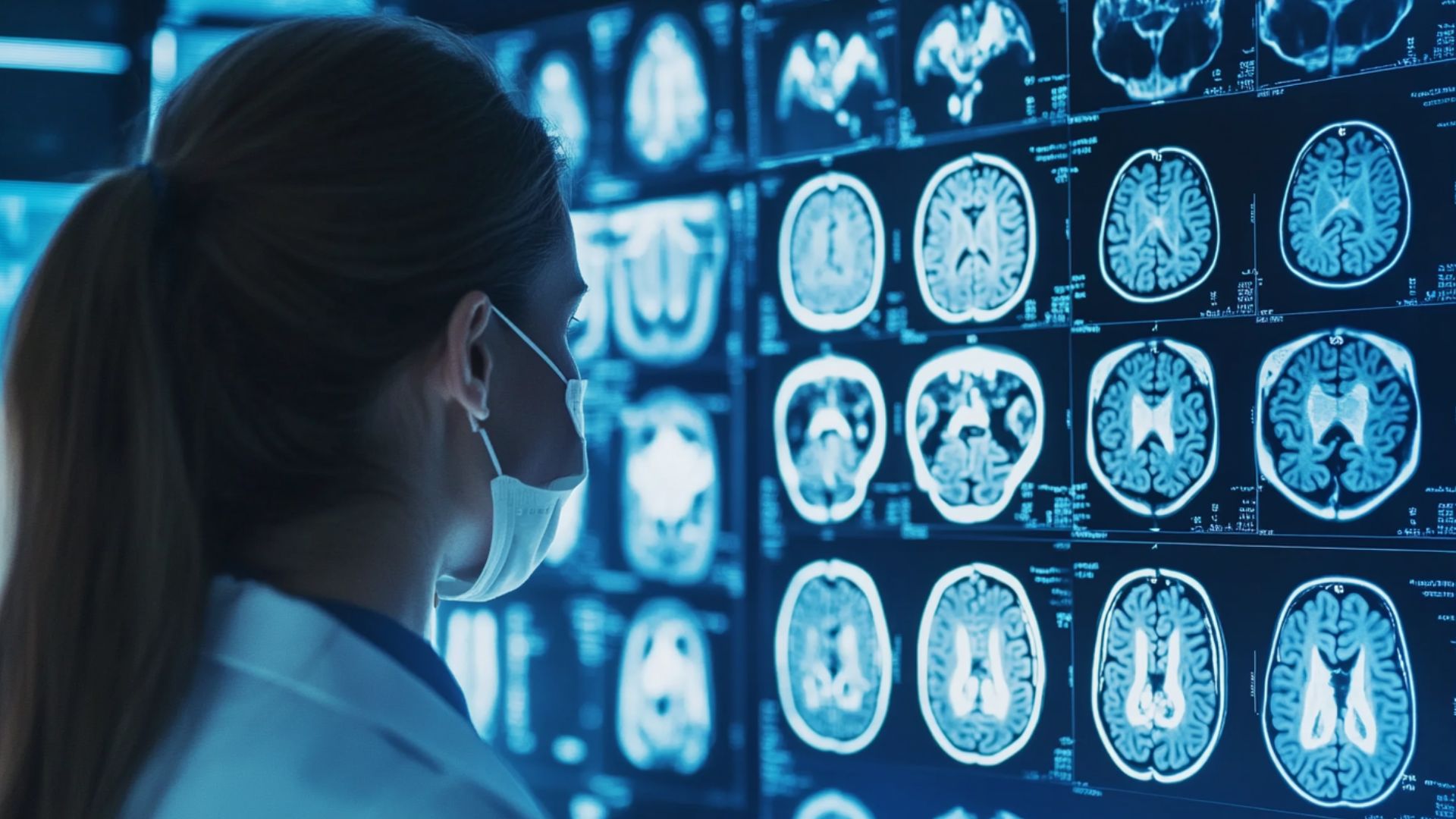
Diagnosing neurological long COVID begins with a thorough medical history linking current symptoms to prior SARS-CoV-2 infection. Clinicians must first exclude other conditions such as anemia, thyroid disorders, and primary psychiatric illness. Long COVID brain fog laboratory testing includes inflammatory markers such as C-reactive protein. Autoantibody panels screen for immune-mediated nerve damage. Vitamin D and B12 levels should be assessed.
Standard diagnostic tests include:
- Cognitive screening: Montreal Cognitive Assessment or Mini-Mental State Examination
- Autonomic function testing: COMPASS-31 questionnaire and tilt-table testing
- Nerve conduction studies: EMG/NCV testing and quantitative sudomotor axon reflex testing
- Neuroimaging: MRI may reveal white matter abnormalities; PET scanning demonstrates reduced cerebral metabolism
- CSF analysis: Lumbar puncture can detect elevated cytokines and oligoclonal bands
- Vestibular evaluation: Videonystagmography helps localize the cause of dizziness
- Skin biopsy: Confirms small-fiber neuropathy
Patients with long COVID brain fog should consult a neurologist if symptoms persist beyond four weeks, progressively worsen, or include weakness, seizures, or visual disturbances. Red flags requiring urgent evaluation include sudden-onset severe headaches, focal numbness, and recurrent falls. Early neurological referral can reduce the risk of permanent disability by 50%. Multidisciplinary clinics provide the most comprehensive care. The CDC recommends specialist consultation for cases that are moderate to severe.
Treatment and Rehabilitation Approaches
Long COVID treatment in neurology remains primarily symptom-based. No single medication reverses the underlying pathology, so care teams combine pharmacotherapy, rehabilitation, and lifestyle modifications. The principle of “start low and go slow” is critical, as overexertion commonly triggers severe symptom exacerbations.
Here are the long COVID treatment in neurology core strategies:
- Cognitive rehabilitation therapy: Brief daily cognitive exercises and attention drills
- Activity pacing: The “spoon theory” approach, with no more than 10% weekly increases
- Volume expansion: 2-3 liters of daily fluid with increased dietary salt
- Compression garments: Waist-high compression stockings and abdominal binders
- Vestibular rehabilitation: Gaze stabilization exercises and balance training
- Pain management: Topical analgesics and transcutaneous electrical nerve stimulation
- Sleep hygiene: Consistent sleep schedule and blue light avoidance
- Mindfulness training: Ten-minute breathing exercises
IVIG therapy shows promise for severe autoantibody-mediated neuropathy. Hyperbaric oxygen therapy may improve cerebral blood flow. Graded exercise therapy must be carefully implemented to avoid post-exertional malaise. Occupational therapists teach energy conservation techniques. Anti-inflammatory nutrition, which emphasizes leafy greens, berries, and nuts, may help reduce systemic inflammation. Daily symptom tracking allows monthly treatment adjustments. Approximately 60% of patients show significant improvement within one year with comprehensive treatment protocols.
Recovery Outlook and the Importance of Early Neurological Care
Recovery from neurological long COVID varies considerably. Approximately 50-70% recover substantially within 1-2 years. Brain fog typically resolves more quickly than peripheral neuropathy. Patients with mild acute COVID-19 generally recover faster, while those with comorbidities experience slower improvement.
Early neurological intervention significantly improves outcomes. Patients who receive specialized care within three months have approximately half the risk of chronic disability. Factors associated with better recovery include a younger age, a mild acute infection, the absence of comorbidities, early specialist referral, treatment adherence, strong support networks, and vaccination status.
Delayed treatment allows neuroinflammation to cause more permanent damage. Symptom diaries and smartphone applications help patients track progress. Multidisciplinary clinics provide coordinated care. COVID-19 vaccination helps maintain recovery gains by preventing reinfection. Patients who pursue early, aggressive treatment are more likely to return to work and resume normal activities. Neurological consultation should lead the care team, as delayed treatment can result in years of preventable disability.










I've given up... the stress her office staff has put me through is just not worth it. You can do so much better, please clean house, either change out your office staff, or find a way for them to be more efficient please. You have to do something. This is not how you want to run your practice. It leaves a very bad impression on your business.
Please, leave your review
Write a comment: