There are many neurological disorders and the cause of their development is often a genetic factor. Understanding this connection is essential for medical research. Genetic factors are understood to be critical in their development and progression. They include Alzheimer’s, Parkinson’s, and multiple sclerosis. Through genetic advances, scientists have uncovered pivotal information. This is how specific gene mutations and variations can influence brain function. It contributes to these diseases. This growing understanding transforms the way we approach neurological illnesses. They range from diagnosis to treatment.
Genetic research is helping to identify biomarkers for early detection. They enable the development of targeted therapies. They address the root causes of these conditions. It paves the way for personalized medicine. There, treatments are generally tailored to an individual’s unique genetic makeup. This field continues to evolve. It promises to revolutionize the treatment. Research will also increase prevention of neurological illnesses. This offers new hope for patients and improves outcomes. It happens through more precise and effective interventions.
The Role of Genetic Mutations in Neurological Diseases
Genetic changes are fundamental in the development of neurological illnesses. These mutations can cause proteins to fold incorrectly and disrupt how cells function. They also interfere with the maintenance of neural structures. It causes the progressive degeneration of nerve cells. Scientists continue to explore the genetic underpinnings of these diseases. They are uncovering critical links between genetic mutations and specific conditions:
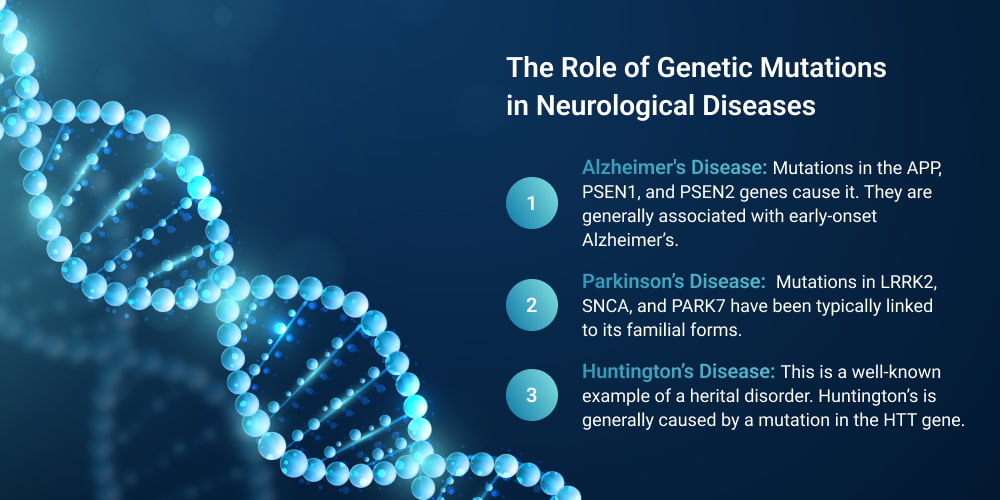
- Alzheimer’s Disease: Mutations in the APP, PSEN1, and PSEN2 genes cause it. They are generally associated with early-onset Alzheimer’s. It leads to the accumulation of amyloid plaques. They disrupt communication between neurons and trigger brain cell death.
- Parkinson’s Disease: Mutations in LRRK2, SNCA, and PARK7 have been typically linked to its familial forms. These mutations affect dopamine-producing neurons. They lead to the motor dysfunction and tremors characteristic of the neurodegenerative disorder.
- Huntington’s Disease: This is a well-known example of a hereditary disorder. Huntington’s is generally caused by a mutation in the HTT gene. It is specifically an expanded CAG repeat. This leads to the production of a toxic protein. They damage brain cells, resulting in movement disorders, cognitive decline, and psychiatric symptoms.
Neurodegenerative Disorders and Genetic Factors
Alzheimer’s and Parkinson’s are heavily influenced by some changes. These diseases involve the progressive degeneration of neurons. They lead to cognitive decline, motor dysfunction, and other debilitating signs. Understanding these changes behind these conditions is crucial. It helps for early diagnosis, risk assessment, and developing targeted therapies. Here are pivotal changes is genetics:
- Alzheimer’s Disease: Inherited mutations in APP, PSEN1, and PSEN2 can cause early-onset. It leads to abnormal protein accumulation (amyloid plaques). They damage brain cells.
- Parkinson’s Disease: LRRK2, SNCA, and PARK7 changes contribute to its familial form. These mutations affect dopamine production. They lead to tremors and rigidity.
Identifying these changes allows for earlier detection. They lead to more accurate predictions of disease progression. Understanding the genetic factors can pave the way for personalized treatments. They target the root causes. Genetic research advances. It offers new hope for potential therapies. They may slow or halt the progression of neurodegenerative disorders. Such therapies improve quality of life for patients.
Hereditary Factors in Neurological Diseases
Heritable factors are significant in the inheritance of many neurological illnesses. Certain conditions run in families. This happens because of genetic changes passed down through generations. These mutations can increase the likelihood of developing disorders. They are often at an early age or in specific families with a history of these diseases. Understanding these hereditary factors is crucial for diagnosis and prevention:
- Huntington’s Disease: This is generally caused by a dominant mutation in the HTT gene. Such a condition is typically inherited in an autosomal dominant pattern. It means that children of affected parents have a 50% chance of inheriting the mutation.
- Amyotrophic Lateral Sclerosis (ALS): Genetic changes in SOD1 and C9orf72 can lead to familial ALS. It significantly increases the risk of developing the neurological disease.
- Parkinson’s Disease: Most cases are sporadic. Mutations in LRRK2 and PARK7 have been generally linked to hereditary forms of Parkinson’s.
Family history is a crucial factor in assessing the risk of neurological illnesses. Genetic testing can help determine the likelihood of inheriting these conditions. Genetic testing identifies mutations. They are typically associated with hereditary factors. It allows for earlier detection and better risk management. This leads to the possibility of personalized treatments to delay or prevent disease onset.
Genome Analysis and Its Impact on Neurological Disease Research
Genetic investigation is a powerful tool in understanding the genetic underpinnings of diseases. It involves the use of advanced technologies. They sequence and analyze the complete genetic code of an individual. This analysis helps identify genetic variations and mutations. They are generally associated with specific conditions. This process helps researchers pinpoint the genes responsible for neurological illnesses. It leads to a deeper understanding of how these conditions develop and progress.
Techniques involved in genome analysis include:
- Whole Genome Sequencing (WGS): This method sequences the entire genome. It provides a comprehensive view of all genetic variations. WGS is crucial for identifying rare mutations linked to complex neurological illnesses.
- Whole Exome Sequencing (WES): This focuses on the protein-coding regions of the genome. WES is often used to identify genetic mutations. They affect gene function. It can lead to severe diseases. These are Huntington’s or ALS.
- Genome-Wide Association Studies (GWAS): These studies compare the genomes of large populations. They identify common genetic variants. These are typically associated with diseases. They help to uncover genetic risk factors for Alzheimer’s and Parkinson’s.
Advances in genome analysis have revolutionized our approach to neurological illnesses. These insights transform personalized medicine. There, treatments are typically tailored to a patient’s unique genetic profile. For instance, targeted therapies can be generally developed to address specific mutations. They offer more effective and less invasive treatment options. Such analyses also enable earlier diagnosis. The integration of genomics into clinical practice is crucial. It reshapes how we treat and manage neurological diseases. It offers new hope for patients.
Identifying Biomarkers for Neurological Diseases Through Genetic Research
Biological markers are crucial in diagnosing, monitoring, and managing diseases. These indicators can be generally found in blood, cerebrospinal fluid, or imaging studies. They provide valuable information about the presence, progression, and severity of a disease. Biomarkers help physicians make more accurate diagnoses and track disease progression. They also allow them tailor treatment plans to individual patients.
Genetic research has been instrumental in identifying and validating these markers. Scientists study the genetic makeup of individuals. They pinpoint specific genetic variations, mutations, or gene expression patterns. They are typically associated with different conditions. Here’s how genetic research contributes to markers in neurological diseases:
- Early Diagnosis: Genetic analysis can identify biological markers. They signal the onset of a disease long before symptoms appear. For example, in Alzheimer’s disease, genetic variations are those in the APOE gene. They can be generally used to assess an individual’s risk. Such variations help to detect the disease at an early, treatable stage.
- Tracking Disease Progression: There are certain markers in the cerebrospinal fluid or blood. They can reflect the extent of neuronal damage or inflammation. For instance, the presence of tau protein tangles in Alzheimer’s patients correlates with cognitive decline. It helps to monitor disease progression over time.
- Predicting Response to Therapies: Genetic biomarkers can also predict how well a patient will respond to them. Individuals with specific mutations in the LRRK2 gene respond differently to dopamine-enhancing drugs. They are typically used in Parkinson’s disease. These biological markers allow for more personalized treatment strategies.
- Monitoring Treatment Efficacy: As treatments become more targeted, biological markers are essential for assessing treatment effectiveness. By tracking changes in genetic markers or protein levels, doctors can adjust treatment plans. They are typically based on how well a patient is responding.
Genetic research changes the identification of biological markers for neurological diseases. They enable earlier, more accurate diagnoses. Such research also improves the ability to predict disease progression. It also customize treatments. Genetic research leads to better patient outcomes.
The Future of Genetic Research in Neurological Disease Treatment
The future of research in treatment holds promise. Emerging technologies like gene therapy and CRISPR drive it. Key trends shaping this future include:
- Gene Therapy: This approach aims to correct or replace defective genes. They are responsible for neurological conditions. Such therapy potentially offers cures for disorders of genetics. These are Huntington’s disease, ALS, and certain forms of inherited dementia.
- CRISPR Technology: It has precision gene-editing capabilities. CRISPR allows for the targeted correction of genetic changes at the DNA level. It offers groundbreaking potential in treating conditions previously deemed incurable.
- Personalized Medicine: Advancements in genetic profiling are pivotal. They will allow treatments tailored to individual genetic makeups. This will make treatments more effective and minimize side effects.
- Stem Cell Research: Using stem cells will help to repair or replace damaged neural tissues. It could open new avenues for treating neurodegenerative diseases. These are Parkinson’s and multiple sclerosis.
Ongoing research is critical to overcoming current challenges. We can look forward to significant breakthroughs. For more information about genetic disorders and available treatments, please reach out to our team.











I've given up... the stress her office staff has put me through is just not worth it. You can do so much better, please clean house, either change out your office staff, or find a way for them to be more efficient please. You have to do something. This is not how you want to run your practice. It leaves a very bad impression on your business.
Please, leave your review
Write a comment: